Brain Scanning Techniques: Neuroimaging Windows into the Mind
Every second, your brain generates enough electrical activity to power a small lightbulb—yet until recently, this incredible neural symphony remained completely invisible to science, locked away behind bone and tissue.
Key Takeaways:
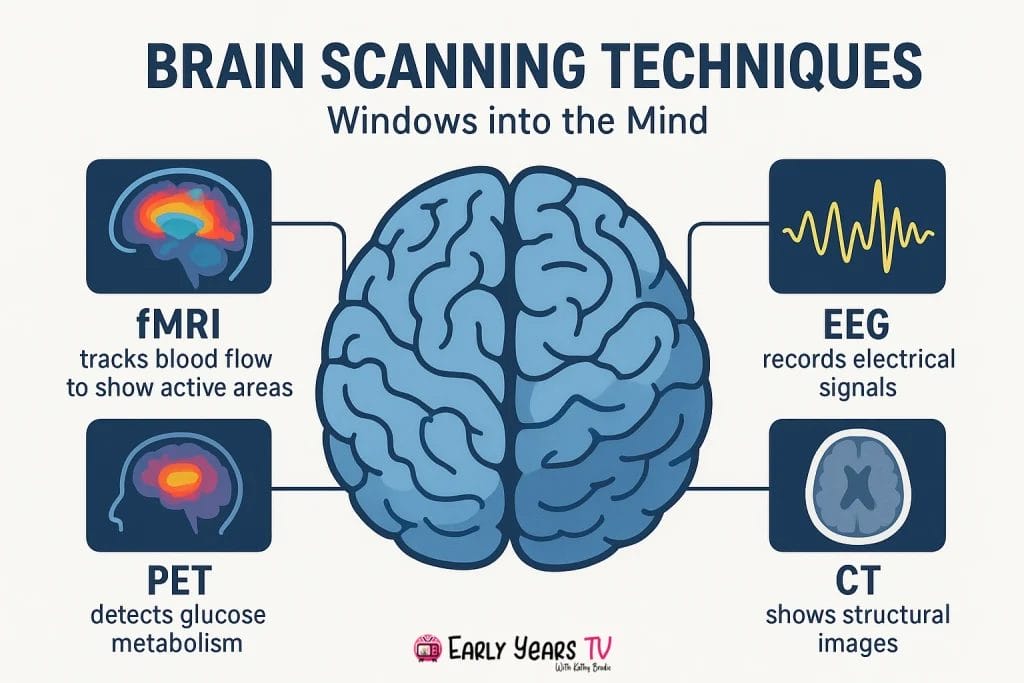
- What are the main brain scanning techniques? The four primary methods are fMRI (detailed brain activity location), EEG (millisecond-precise neural timing), PET (metabolism tracking), and structural MRI (anatomical imaging), each serving different research and clinical purposes.
- Which brain scan is best for my needs? fMRI excels for research requiring spatial precision, EEG works best for timing-critical studies or epilepsy diagnosis, while structural MRI provides optimal brain health screening and surgical planning guidance.
- How do I choose the right technique? Match your research question to technique strengths: “where” questions need fMRI spatial resolution, “when” questions require EEG temporal precision, and connectivity studies benefit from DTI pathway mapping.
Introduction
Brain scanning technology has revolutionized our understanding of the human mind, transforming neuroscience from a field limited to studying deceased brains to one that can observe living neural activity in real-time. These remarkable tools allow researchers and clinicians to peer inside the skull without making a single incision, revealing how our brains process thoughts, emotions, and memories as they happen.
Modern neuroimaging techniques have unlocked discoveries that seemed impossible just decades ago. We can now watch neural networks activate when children learn to read, observe how different brain regions coordinate during complex thinking, and track the development of critical cognitive skills from infancy through adulthood. This research has profound implications for understanding normal brain development, as explored in our comprehensive guide to neuroscience and early brain development, and has transformed how we approach everything from education to mental health treatment.
Whether you’re a student learning about the brain, a patient preparing for a scan, or simply curious about how these technologies work, understanding brain scanning techniques provides valuable insight into one of science’s most exciting frontiers. This guide will walk you through the major scanning methods, help you understand when each technique is most useful, and explore the real-world applications that are changing lives every day. From the millimeter precision of fMRI to the split-second timing of EEG, each technique offers a unique window into the extraordinary complexity of human cognition.
Understanding Brain Imaging Fundamentals
What Makes Brain Scanning Possible
Brain scanning techniques work by detecting and measuring different types of activity within the living brain. Unlike X-rays that simply show bone structure, neuroimaging methods can reveal dynamic processes—the electrical signals neurons use to communicate, changes in blood flow that indicate active brain regions, and even the physical structure of neural pathways.
The key insight enabling modern brain scanning is that brain activity leaves measurable traces. When neurons fire, they consume oxygen and glucose, causing local blood flow changes. They also generate tiny electrical fields that can be detected at the scalp. Some techniques measure the magnetic fields these electrical currents create, while others track how different brain tissues respond to powerful magnetic fields.
Each scanning method essentially captures a different “signature” of brain activity. This is why neuroscientists often combine multiple techniques—what they call “multimodal imaging”—to get a complete picture. The brain operates across multiple timescales simultaneously, from the millisecond firing of individual neurons to the minutes-long processes involved in memory development and learning.
Understanding these different signatures helps explain why no single brain scanning technique can answer every research question. Each method involves trade-offs between spatial precision (how accurately we can locate brain activity) and temporal precision (how precisely we can time when activity occurs). Modern neuroscience success comes from strategically choosing the right tool for each specific research question.
Types of Brain Activity We Can Measure
Brain scanning techniques fall into several categories based on what type of neural activity they detect. Understanding these categories helps explain why different techniques excel in different situations and why researchers often use multiple methods to study the same question.
Electrical activity methods directly measure the electrical signals that neurons use to communicate. These techniques, including EEG and its variants, offer exceptional timing precision because they detect the actual electrical activity as it happens. However, the skull and surrounding tissues distort these electrical signals, making it challenging to pinpoint exactly where in the brain the activity originates.
Hemodynamic methods measure changes in blood flow and oxygenation that accompany neural activity. When brain regions become active, they demand more oxygen and nutrients, causing measurable changes in local blood flow. Techniques like fMRI capitalize on this relationship, offering excellent spatial precision but with a time delay of several seconds as the blood supply adjusts to neural demands.
Metabolic and structural methods examine brain tissue properties, chemical compositions, or anatomical features. These approaches can reveal brain damage, track disease progression, or map the physical connections between brain regions. While they may not show real-time activity, they provide crucial context for understanding brain function.
| Signal Type | What It Measures | Timing | Spatial Precision | Main Techniques |
|---|---|---|---|---|
| Electrical | Direct neural firing | Milliseconds | Moderate | EEG, MEG |
| Hemodynamic | Blood flow changes | Seconds | High | fMRI, PET |
| Structural | Brain anatomy | Static | Highest | MRI, CT |
| Metabolic | Chemical activity | Minutes | High | PET, MRS |
Major Brain Scanning Techniques Explained
Functional Magnetic Resonance Imaging (fMRI)
Functional Magnetic Resonance Imaging represents one of neuroscience’s most powerful tools for understanding brain function. By detecting blood oxygen changes that accompany neural activity, fMRI can pinpoint active brain regions with remarkable precision while participants perform cognitive tasks, watch movies, or even rest quietly.
The technology works through a clever biological hack. When neurons become active, they consume oxygen from surrounding blood vessels. The brain compensates by flooding the active area with fresh, oxygen-rich blood—actually delivering more oxygen than the neurons need. This creates a detectable change in the magnetic properties of blood in that region, which the fMRI scanner can measure.
fMRI’s greatest strength lies in its spatial resolution. Modern scanners can distinguish activity differences in brain regions just 1-3 millimeters apart, allowing researchers to map specific functions to particular brain areas with extraordinary precision. This capability has revolutionized our understanding of brain organization, revealing that many cognitive abilities we take for granted actually involve precise coordination between multiple, specialized brain regions.
However, fMRI’s temporal limitations create important constraints. Because the blood oxygen response takes 4-6 seconds to peak after neural activity begins, fMRI cannot capture the rapid, millisecond-scale processes that characterize much of brain function. This makes fMRI ideal for studying which brain regions are involved in specific tasks, but less suitable for understanding the precise timing of neural events.
Research using fMRI has transformed our understanding of cognitive development, particularly in areas like executive function development where scientists can track how prefrontal cortex networks mature throughout childhood and adolescence. These studies reveal that many cognitive abilities we expect children to master actually depend on brain regions that continue developing well into the twenties.
Clinical applications of fMRI include pre-surgical planning for brain tumor removal, where surgeons need to identify critical language and motor areas to preserve during surgery. The technique also plays increasingly important roles in psychiatric research, helping identify brain differences associated with conditions like depression, anxiety, and autism spectrum disorders.
Electroencephalography (EEG)
Electroencephalography offers a fundamentally different approach to brain scanning by directly measuring the electrical activity of neurons through electrodes placed on the scalp. This direct measurement provides unparalleled temporal precision, capturing brain activity changes within milliseconds of when they actually occur.
EEG works by detecting the synchronized electrical activity of thousands of neurons near the brain’s surface. When large groups of neurons fire together, they create electrical fields strong enough to be measured through the skull and scalp. Modern EEG systems use 64, 128, or even 256 electrodes to capture this activity across the entire head, creating detailed maps of electrical brain activity over time.
The millisecond precision of EEG makes it invaluable for studying the timing of cognitive processes. Researchers can present a stimulus—like a word or image—and track exactly when different brain regions respond, revealing the sequence of neural events underlying perception, attention, and decision-making. This temporal precision has proven crucial for understanding attention span development in children, where scientists can measure exactly when and how attentional brain networks mature.
EEG’s main limitation involves spatial resolution. Because electrical signals blur as they pass through the skull, pinpointing the exact source of brain activity can be challenging. While sophisticated mathematical techniques can improve source localization, EEG generally cannot match fMRI’s spatial precision. Additionally, EEG primarily detects activity from brain areas near the surface, making it less sensitive to deep brain structures.
Clinical applications of EEG extend far beyond research settings. Neurologists routinely use EEG to diagnose epilepsy, monitor seizure activity, and assess brain function in patients with head injuries or neurological disorders. The technique’s portability and relatively low cost make it accessible in many healthcare settings where fMRI would be impractical.
Recent technological advances have made EEG increasingly practical for diverse applications. Wireless EEG systems allow natural movement during recording, while consumer-grade devices bring basic brain monitoring capabilities to homes and schools. These developments open exciting possibilities for real-time neurofeedback training and personalized learning approaches.
Event-Related Potentials (ERP)
Event-Related Potentials represent a specialized application of EEG technology that has become indispensable for understanding the timing of cognitive processes. By averaging EEG responses across many identical stimuli, ERPs reveal the brain’s consistent electrical responses to specific events, creating detailed maps of neural processing stages.
The ERP methodology involves presenting the same stimulus many times—typically 50-200 repetitions—while recording EEG activity. Random brain activity cancels out through averaging, while consistent responses to the stimulus become clearly visible. This process reveals distinct components that occur at specific times after stimulus presentation, each reflecting different stages of cognitive processing.
ERP components are typically named for their polarity (positive or negative) and timing. The P300 component, for example, represents a positive electrical response occurring about 300 milliseconds after stimulus presentation. This component reflects attention and memory processes, making it valuable for studying cognitive development and neurological conditions. Other important components include the N170 (face processing), N400 (language comprehension), and P600 (syntactic processing).
The cognitive insights from ERP research have been particularly valuable for understanding language processing. Studies of language-related ERP components have revealed how the brain distinguishes between meaningful and nonsensical words, processes grammatical violations, and integrates meaning across sentences. This research directly connects to our understanding of language disorders, as discussed in our guide to Broca’s vs Wernicke’s aphasia, where modern neuroimaging has refined our understanding of how language networks function.
ERP methodology allows researchers to study cognitive processes that occur too quickly for other brain scanning techniques to capture. While fMRI might reveal which brain regions are active during language processing, ERPs can show the precise timing of different processing stages—when the brain first recognizes a word, when it accesses meaning, and when it integrates that meaning with context.
Clinical applications of ERPs include assessment of cognitive function in patients who cannot respond verbally, evaluation of hearing in infants, and monitoring cognitive changes in aging or neurological disease. The technique’s sensitivity to subtle processing differences makes it valuable for early detection of cognitive decline and for tracking treatment effectiveness.
Other Important Techniques
Several additional neuroimaging techniques complement the major methods described above, each offering unique capabilities for specific research questions and clinical applications. Understanding these techniques provides a complete picture of the modern neuroimaging toolkit.
Positron Emission Tomography (PET) measures brain activity by tracking radioactive tracers injected into the bloodstream. Different tracers can reveal blood flow, glucose metabolism, or the distribution of specific neurotransmitters. While PET provides excellent spatial resolution and unique metabolic information, the radiation exposure and complex logistics limit its use, particularly in healthy volunteers and children.
Magnetoencephalography (MEG) detects the magnetic fields generated by electrical brain activity, offering both excellent temporal precision (like EEG) and better spatial localization. MEG systems require specialized, magnetically shielded rooms and are extremely expensive, but they provide unparalleled combinations of timing and location information for studying rapid cognitive processes.
Diffusion Tensor Imaging (DTI) maps the brain’s white matter pathways by tracking how water molecules move along nerve fibers. This technique reveals the “highways” connecting different brain regions, providing insights into brain connectivity and development. DTI has been particularly valuable for understanding how neural networks mature during childhood and how brain injuries affect communication between regions.
Near-Infrared Spectroscopy (NIRS) represents an emerging portable alternative for measuring brain activity through changes in blood oxygenation. While less precise than fMRI, NIRS systems can be wireless and allow natural movement, making them particularly valuable for studying infant brain development and real-world cognitive processes.
Recent advances in portable brain scanning devices are democratizing neuroimaging research. Companies now manufacture EEG headsets costing hundreds rather than thousands of dollars, while portable fMRI and ultrasound-based brain imaging systems are entering clinical trials. These developments promise to make brain scanning accessible in schools, homes, and community settings previously beyond the reach of traditional neuroimaging.
| Technique | Spatial Resolution | Temporal Resolution | Main Strengths | Primary Limitations | Typical Uses |
|---|---|---|---|---|---|
| fMRI | 1-3mm | 2-6 seconds | Excellent spatial precision, non-invasive | Poor temporal resolution, expensive | Cognitive studies, pre-surgical planning |
| EEG | 1-2cm | Milliseconds | Excellent temporal resolution, portable | Limited spatial precision, surface only | Epilepsy diagnosis, cognitive timing |
| ERP | 1-2cm | Milliseconds | Precise cognitive timing, specific processes | Limited spatial precision, many trials needed | Language research, cognitive assessment |
| PET | 4-6mm | Minutes | Metabolic information, neurotransmitter mapping | Radiation exposure, expensive | Research only, receptor studies |
| MEG | 2-5mm | Milliseconds | Good spatial and temporal resolution | Extremely expensive, limited availability | Research centers only |
| DTI | 1-2mm | Static | White matter pathways, connectivity | No temporal information, complex analysis | Brain development, injury assessment |
Choosing the Right Brain Scanning Method
Research Question Determines Technique
The fundamental principle guiding neuroimaging research is that the research question must drive technique selection, not the other way around. Each brain scanning method excels at answering specific types of questions while being poorly suited for others. Understanding these strengths and limitations is crucial for designing effective studies and interpreting results appropriately.
Questions about location (“Where in the brain does this happen?”) naturally favor techniques with high spatial resolution. If researchers want to identify which specific brain regions activate during mathematical problem-solving, fMRI would be the optimal choice due to its millimeter-scale precision. However, if the question instead focuses on timing (“When does the brain first detect a face?”), EEG or MEG would be more appropriate despite their limited spatial precision.
Questions about development and change require techniques capable of tracking differences across age groups or over time. Longitudinal studies following children as they develop often rely on fMRI because it can safely be repeated many times without radiation exposure. However, studying very young children may require techniques like NIRS that can tolerate movement, or EEG protocols specifically designed for pediatric populations.
Questions about connectivity and networks increasingly drive modern neuroscience research. How do different brain regions communicate with each other? How do these networks change with age, learning, or disease? These questions require techniques that can either measure connectivity directly (like DTI for structural connections) or sophisticated analysis methods that infer functional connectivity from synchronized activity patterns across brain regions.
Clinical research questions add additional constraints. Studies involving patient populations must consider safety limitations, comfort requirements, and practical feasibility. Patients with metal implants cannot undergo MRI scanning, while individuals with severe claustrophobia may struggle with the enclosed scanner environment. Pediatric research requires child-friendly protocols and often benefits from faster scanning techniques that minimize motion artifacts.
The growing integration of multiple techniques within single studies reflects recognition that complex research questions require complementary approaches. A complete understanding of language development, for instance, might combine fMRI to identify active brain regions, EEG to measure the timing of language processing, and DTI to map developing language pathways.
| Research Question Type | Optimal Primary Technique | Supporting Techniques | Example Study |
|---|---|---|---|
| “Where does X happen?” | fMRI | EEG for timing | Locating mathematical processing areas |
| “When does Y occur?” | EEG/MEG | fMRI for location | Timing of face recognition |
| “How do regions connect?” | DTI | fMRI for functional connectivity | Language network development |
| “How does Z change with age?” | Longitudinal fMRI | Behavioral assessments | Working memory development |
| “Is this brain region damaged?” | Structural MRI | Clinical assessment | Post-stroke evaluation |
Practical Considerations
Beyond scientific requirements, numerous practical factors influence technique selection in both research and clinical settings. These considerations often prove as important as the scientific requirements in determining which brain scanning approach is most appropriate for a given situation.
Cost and accessibility represent major practical constraints. fMRI scanners cost millions of dollars and require specialized facilities with magnetic shielding, helium cooling systems, and expert technical support. In contrast, EEG systems can cost as little as tens of thousands of dollars and operate in standard laboratory or clinical spaces. This cost difference means that while fMRI provides superior spatial resolution, EEG remains the most widely accessible neuroimaging technique globally.
Patient comfort and safety requirements vary dramatically across techniques. fMRI scanning requires participants to lie still in a narrow, noisy tube for 30-90 minutes, which can be challenging for individuals with claustrophobia, chronic pain, or movement disorders. EEG involves placing electrodes on the scalp but allows natural sitting positions and even movement with wireless systems. For pediatric populations, the ability to have a parent present and the option for shorter protocols often outweighs technical advantages of more sophisticated techniques.
Time constraints affect both research efficiency and clinical practicality. A complete fMRI session including setup and multiple scans typically requires 2-3 hours, while basic EEG recordings can be completed in 30-60 minutes. For clinical applications where rapid diagnosis is crucial, techniques that provide faster results may be preferred even if they offer less detailed information.
Motion sensitivity creates particular challenges for certain populations. fMRI requires participants to remain very still because even small head movements can create artifacts that obscure real brain activity. This makes fMRI challenging for young children, individuals with neurological conditions affecting movement, or anyone who struggles to remain motionless for extended periods. EEG is more tolerant of movement, though excessive motion still creates artifacts.
Safety considerations extend beyond immediate scanning risks to long-term research participation. While all modern neuroimaging techniques are considered safe for research use, repeated radiation exposure from PET scans limits how often individuals can participate in studies. The FDA provides comprehensive guidelines for medical imaging safety that inform research protocols and clinical practices.
Clinical Applications and Real-World Uses
Diagnostic Applications
Brain scanning techniques have transformed medical diagnosis by providing non-invasive windows into neurological and psychiatric conditions. These applications demonstrate how research advances translate into improved patient care and clinical outcomes across multiple medical specialties.
Brain tumor detection and monitoring represents one of the most established clinical applications of neuroimaging. MRI scans can detect tumors as small as a few millimeters, while specialized techniques like diffusion imaging reveal how tumors affect surrounding brain tissue. fMRI helps surgeons identify critical brain areas that must be preserved during tumor removal, dramatically improving surgical outcomes and reducing post-operative complications.
Epilepsy localization relies heavily on EEG monitoring to identify seizure origins within the brain. Continuous EEG monitoring in specialized epilepsy units can record seizure activity as it occurs, helping neurologists pinpoint the specific brain regions where abnormal electrical activity begins. This information proves crucial for patients considering surgical treatment, where successful outcomes depend on accurate identification of seizure sources.
Stroke assessment and recovery tracking benefits from multiple neuroimaging approaches working together. Initial CT scans can quickly identify bleeding or blood clots, while MRI provides detailed information about brain tissue damage. Follow-up scans track recovery progress and help guide rehabilitation strategies. Advanced techniques like DTI can reveal damage to white matter pathways that might not be visible on standard scans but significantly affect recovery potential.
Psychiatric diagnosis increasingly incorporates neuroimaging findings, though clinical applications remain more limited than in neurological conditions. Research has identified brain differences associated with depression, schizophrenia, autism spectrum disorders, and attention-deficit hyperactivity disorder. While these findings rarely provide definitive diagnostic information on their own, they contribute to comprehensive clinical assessments and help guide treatment planning.
The integration of neuroimaging with clinical decision-making continues expanding as techniques become more accessible and analysis methods improve. Many medical centers now routinely use brain scanning to monitor treatment effectiveness, adjust medication dosages, and predict recovery outcomes. Expert neuroscience educators like those featured in our professional video content emphasize how these advances are changing clinical practice across multiple disciplines.
Treatment Planning and Monitoring
Modern medicine increasingly relies on brain scanning techniques not just for diagnosis, but for planning treatments and monitoring their effectiveness. This represents a shift toward personalized medicine where treatment decisions are informed by individual brain characteristics and responses.
Pre-surgical mapping has become standard practice for brain surgery patients. Surgeons use fMRI to identify language areas, motor regions, and other critical brain functions before operating near these sensitive areas. This mapping process, sometimes called “functional neurosurgery,” allows surgeons to remove tumors or treat epilepsy while minimizing risks to essential brain functions. The precision of modern pre-surgical mapping has dramatically reduced complications and improved quality of life outcomes for neurosurgical patients.
Therapy effectiveness assessment uses brain scanning to track how treatments change brain activity over time. For example, cognitive-behavioral therapy for depression can be monitored using fMRI to see whether treatment normalizes activity in brain regions associated with mood regulation. Speech therapy for stroke patients can be tracked using EEG to monitor recovery of language-related brain responses. These objective measures complement traditional clinical assessments and help optimize treatment duration and intensity.
Recovery monitoring protocols provide crucial information about healing progress following brain injuries. Serial MRI scans can track tissue recovery, swelling reduction, and the development of compensatory brain activity as patients regain function. DTI can reveal whether white matter pathways are healing properly, while functional scanning can show how the brain reorganizes to compensate for damaged areas.
The concept of brain plasticity—the brain’s ability to reorganize and adapt—underlies many treatment monitoring applications. Historical research, including landmark studies like Roger Sperry’s split-brain research, established that the brain shows remarkable capacity for adaptation following injury. Modern neuroimaging allows clinicians to observe this plasticity directly, guiding rehabilitation strategies and providing prognostic information for patients and families.
Medication monitoring represents an emerging application where brain scanning helps optimize pharmaceutical treatments. PET scanning can reveal how medications affect neurotransmitter systems, while fMRI can track whether drugs successfully normalize abnormal brain activity patterns. This approach shows particular promise for psychiatric medications, where current prescribing practices often involve trial-and-error approaches that could potentially be guided by objective brain measures.
Research Design and Interpretation Guidelines
Planning Effective Neuroimaging Studies
Successful neuroimaging research requires careful planning that balances scientific rigor with practical constraints. The complexity and cost of brain scanning studies make thorough preparation essential for obtaining meaningful results while using resources efficiently.
Sample size considerations in neuroimaging research often differ from traditional psychological studies. While behavioral studies might require hundreds of participants to detect small effects, neuroimaging studies sometimes achieve adequate statistical power with smaller samples due to the rich data each participant provides. However, this advantage comes with caveats—individual differences in brain anatomy can create substantial variability, and artifact rejection may reduce usable data below initial sample sizes.
Modern neuroimaging research increasingly emphasizes larger sample sizes following recognition that many early studies were underpowered. The movement toward “big data” neuroimaging, exemplified by projects collecting data from thousands of participants, reflects growing awareness that robust findings require substantial sample sizes despite the detailed measurements each technique provides.
Control group requirements demand particular attention in neuroimaging research. Unlike behavioral studies where controls and experimental groups can be matched on observable characteristics, brain scanning studies must consider factors like head size, brain anatomy variations, and even genetic differences that can affect neuroimaging signals. Age-matched controls become crucial when studying developmental populations, while healthy controls must be carefully screened to ensure absence of neurological or psychiatric conditions.
Avoiding common pitfalls requires understanding the specific challenges each technique presents. Motion artifacts plague fMRI studies, particularly with children or clinical populations. Solutions include shorter scan protocols, motion monitoring systems, and specialized analysis techniques that can correct for small movements. EEG studies must control for electrical artifacts from muscle activity, eye movements, and environmental electrical noise through careful electrode placement and analysis procedures.
Statistical considerations in neuroimaging involve unique challenges due to the “multiple comparisons problem.” A single brain scan contains hundreds of thousands of data points, each representing activity in a small brain region. Testing each point for statistical significance creates enormous multiple comparisons challenges that require special correction procedures. Standard statistical approaches often prove inadequate, leading to development of specialized neuroimaging analysis methods.
Interpreting Results Accurately
Accurate interpretation of neuroimaging results requires understanding both the capabilities and limitations of each technique, along with the broader context of brain function and individual differences. Misinterpretation of brain scanning data can lead to overconfident conclusions that don’t reflect the true state of scientific knowledge.
Statistical significance versus clinical significance represents a crucial distinction often overlooked in neuroimaging interpretation. A brain region showing statistically significant activation during a task may reflect a genuine neural response, but whether this response is large enough or consistent enough to have clinical relevance requires separate evaluation. Effect sizes and confidence intervals provide important context for judging practical significance beyond statistical thresholds.
Multiple comparison corrections are essential but complex in neuroimaging research. When testing thousands of brain regions simultaneously, some will appear significant by chance alone. Various correction methods exist, from conservative approaches that minimize false positives to more liberal methods that reduce false negatives. The choice of correction method can dramatically affect results and conclusions, making transparency about analysis decisions crucial for interpretation.
Publication bias awareness affects neuroimaging literature just as it does other research areas. Studies showing clear, significant brain activation differences are more likely to be published than studies finding no differences. This bias can create false impressions about how consistently certain brain regions are involved in specific tasks. Recent meta-analyses of neuroimaging studies have highlighted the importance of considering publication bias when interpreting research findings.
Individual differences in brain anatomy and function create important interpretation challenges. What appears as “abnormal” brain activity in one individual might fall within the normal range when individual differences are properly considered. Factors like age, sex, handedness, education level, and cultural background all influence brain structure and function in ways that can affect neuroimaging results.
Reproducibility considerations have become increasingly important as the neuroimaging field matures. Early neuroimaging studies often had small sample sizes and flexible analysis approaches that could lead to spurious findings. Current best practices emphasize pre-registered analysis plans, larger sample sizes, and replication attempts to ensure findings represent genuine brain phenomena rather than statistical artifacts.
The interpretation of neuroimaging results also requires careful consideration of the relationship between brain activity and behavior. Observing brain activation during a task doesn’t necessarily mean that brain region is essential for the task—it might reflect incidental processes, compensatory activity, or even effort-related responses. Converging evidence from multiple techniques, patient studies, and behavioral research provides stronger foundations for causal claims about brain-behavior relationships.
Ethical Considerations and Future Directions
Privacy and Consent in Brain Imaging
Brain imaging research raises unique ethical considerations that extend beyond traditional research ethics to encompass questions about mental privacy, data ownership, and the implications of increasingly sophisticated technologies that can peer into human thoughts and emotions.
Incidental findings protocols address what happens when brain scans reveal unexpected abnormalities. Unlike other medical tests where patients seek scans specifically to investigate suspected problems, research participants typically have no expectation that scanning will reveal medical issues. When scans do detect potential abnormalities—which occurs in approximately 2-8% of healthy research participants—researchers face complex decisions about whether and how to inform participants.
Current best practices require pre-established protocols for handling incidental findings, with participants informed during consent about the possibility of discovering unexpected abnormalities. Some studies employ medical professionals to review all scans for potential clinical significance, while others limit review to obvious, serious abnormalities. The challenge lies in balancing participants’ right to know about their health with the reality that many apparent abnormalities on research scans prove clinically insignificant.
Data sharing and anonymization present evolving challenges as neuroimaging databases grow larger and more interconnected. Brain scans contain extraordinarily detailed information about individual brain structure and function that could potentially be used to identify specific individuals. Traditional anonymization methods that simply remove names and obvious identifiers may prove inadequate for protecting privacy in brain imaging data.
Recent research has demonstrated that brain scans can serve as “neural fingerprints” that uniquely identify individuals even when traditional identifying information is removed. This capability raises concerns about data security and the potential for re-identification of supposedly anonymous research participants. Developing effective anonymization methods while preserving scientific value represents an ongoing challenge for the neuroimaging community.
Informed consent complexities in brain imaging extend beyond typical research considerations to include questions about data ownership, future use of brain images, and the implications of discovering information about personality, cognitive abilities, or disease risk. Participants may not fully understand what brain scanning can reveal about them or how this information might be used in the future.
Emerging Technologies and Trends
The future of brain scanning technology promises even more remarkable capabilities while potentially democratizing access to neuroimaging tools that were once available only in specialized research centers. These developments could transform both scientific research and clinical practice over the coming decade.
Artificial intelligence-enhanced imaging analysis represents perhaps the most significant current advancement in neuroimaging. Machine learning algorithms can now detect patterns in brain scans that human experts might miss, potentially enabling earlier detection of neurological diseases, more accurate diagnosis of psychiatric conditions, and personalized treatment predictions. AI systems have shown promise in analyzing brain scans for early signs of Alzheimer’s disease, predicting treatment responses in depression, and identifying subtle brain differences associated with autism spectrum disorders.
However, AI-enhanced analysis also raises important questions about interpretability and bias. When machine learning systems make predictions based on brain scans, understanding how they reach their conclusions can be challenging. Ensuring that AI systems work fairly across different populations and don’t perpetuate existing healthcare disparities remains an active area of development.
Portable brain scanning developments are making neuroimaging accessible outside traditional laboratory and hospital settings. Companies are developing portable MRI scanners that could be transported to patients rather than requiring patients to travel to specialized centers. Wearable EEG devices now provide research-quality brain monitoring in natural environments, while ultrasound-based brain imaging systems promise non-invasive, portable alternatives to traditional neuroimaging.
These portable technologies could revolutionize how we study brain development in natural settings, enabling research in schools, homes, and community environments where children’s brains actually develop. The implications for understanding real-world cognitive processes and for providing neuroimaging access in underserved communities could be profound.
Real-time neurofeedback applications combine brain scanning with immediate feedback to participants about their brain activity. This approach shows promise for treating attention problems, anxiety, depression, and various neurological conditions. Participants can learn to modify their brain activity patterns through practice and feedback, potentially providing non-pharmaceutical treatment options for various conditions.
The convergence of brain scanning with other emerging technologies opens exciting possibilities. Brain-computer interfaces that translate neural signals into computer commands could transform assistive technology for individuals with paralysis or communication disorders. Virtual reality combined with neuroimaging creates unprecedented opportunities for studying brain responses to immersive experiences and for providing therapeutic interventions in controlled virtual environments.
As these technologies mature, questions about accessibility, regulation, and appropriate use become increasingly important. The democratization of brain scanning could provide enormous benefits for research and healthcare, but it also requires careful consideration of how to ensure quality, protect privacy, and prevent misuse of powerful technologies that reveal intimate details about human cognition and emotion.
Conclusion
Brain scanning techniques have revolutionized our understanding of the human mind, transforming neuroscience from studying static brain tissue to observing living neural networks in real-time. From fMRI’s millimeter precision to EEG’s split-second timing, each technique offers unique windows into cognitive processes that were once completely mysterious.
The choice of brain scanning method depends entirely on your research question—whether you need to know where brain activity occurs, when it happens, or how different regions connect. While fMRI excels at pinpointing active brain areas, EEG captures the rapid timing of neural events, and techniques like DTI reveal the brain’s structural highways.
As these technologies become more accessible and AI-enhanced analysis grows more sophisticated, brain scanning will likely extend beyond research laboratories into schools, clinics, and even homes. Understanding these techniques helps us appreciate both the remarkable complexity of human cognition and the ingenious methods scientists use to unlock the brain’s secrets.
Frequently Asked Questions
What are the main types of brain scans?
The four main types of brain scans are fMRI (functional Magnetic Resonance Imaging), EEG (Electroencephalography), PET (Positron Emission Tomography), and structural MRI. fMRI measures blood flow changes to detect brain activity with excellent spatial precision. EEG records electrical brain activity with millisecond timing accuracy. PET tracks radioactive tracers to show metabolism and neurotransmitter activity. Structural MRI reveals detailed brain anatomy and can detect tumors, injuries, or developmental differences.
What are the 5 technologies used to scan the brain?
The five key brain scanning technologies are fMRI, EEG, PET, MEG (Magnetoencephalography), and DTI (Diffusion Tensor Imaging). fMRI detects blood oxygen changes indicating neural activity. EEG measures electrical brain signals through scalp electrodes. PET uses radioactive tracers to show brain metabolism. MEG detects magnetic fields from neural electrical activity. DTI maps white matter pathways by tracking water molecule movement along nerve fibers, revealing brain connectivity.
What is the best brain imaging technique?
No single brain imaging technique is “best”—the optimal choice depends on your specific research question or clinical need. fMRI offers excellent spatial resolution for locating brain activity but poor timing precision. EEG provides millisecond timing but limited spatial accuracy. For surgical planning, fMRI excels at mapping critical brain areas. For epilepsy diagnosis, EEG captures seizure timing. For connectivity research, DTI reveals structural pathways. The most comprehensive studies often combine multiple techniques.
What is the best way to scan your brain?
The best brain scanning approach depends on your specific purpose. For general brain health screening, structural MRI provides detailed images of brain anatomy to detect tumors, strokes, or injuries. For research participation, techniques vary by study requirements. fMRI suits cognitive studies but requires lying still for extended periods. EEG works better for those uncomfortable with enclosed spaces. Always consult qualified medical professionals who can recommend appropriate scanning based on your individual needs and circumstances.
How safe are brain scans?
Modern brain scanning techniques are very safe when performed properly. MRI and fMRI use magnetic fields and radio waves without ionizing radiation, making them safe for repeated use. EEG only measures electrical activity and poses no known risks. PET involves small amounts of radioactivity but exposure levels are carefully controlled and considered safe for medical use. Pregnant women should avoid certain scans, and people with metal implants cannot undergo MRI. Always inform technicians about medical conditions or implants.
How long does a brain scan take?
Brain scan duration varies significantly by technique and purpose. Structural MRI scans typically take 30-60 minutes. fMRI sessions last 45-90 minutes including setup and multiple scans. EEG recordings range from 30 minutes to several hours depending on the study. PET scans require 1-3 hours including tracer injection and waiting periods. Quick emergency CT scans take just 5-10 minutes. Research studies often involve longer sessions to collect sufficient data for analysis.
Can brain scans detect mental illness?
Brain scans currently cannot definitively diagnose most mental illnesses, but they provide valuable supporting information. Research has identified brain differences associated with depression, schizophrenia, ADHD, and autism, but these patterns aren’t consistent enough for diagnostic purposes. Scans help rule out physical causes of symptoms, monitor treatment effects, and guide therapy planning. Mental illness diagnosis still relies primarily on clinical interviews, behavioral observations, and psychological assessments rather than brain imaging alone.
What do brain scans show about learning?
Brain scans reveal fascinating insights about learning processes, showing how different brain regions activate during skill acquisition and how neural networks strengthen with practice. fMRI studies demonstrate that learning new information initially requires widespread brain activation, but becomes more efficient and localized as skills develop. EEG research reveals that successful learning involves specific brainwave patterns and timing. These findings help educators understand why repetition strengthens learning and how individual differences in brain development affect educational approaches.
References
American Academy of Pediatrics. (2019). Guidelines for attention span development in children. Pediatrics, 143(2), e20182948.
Baddeley, A., & Hitch, G. (1974). Working memory. Psychology of Learning and Motivation, 8, 47-89.
Bauer, P. J. (2007). Remembering the Times of Our Lives: Memory in Infancy and Beyond. Lawrence Erlbaum Associates.
Donaldson, M. (1992). Human Minds: An Exploration. Allen Lane.
Food and Drug Administration. (2024). Medical imaging safety guidelines. FDA Radiation-Emitting Products.
Gardner, H. (2018). Multiple Intelligences: New Horizons in Theory and Practice. Basic Books.
Gathercole, S. E., & Pickering, S. J. (2000). Working Memory Deficits in Children with Learning Difficulties. Academic Press.
Gazzaniga, M. S. (2015). Tales from Both Sides of the Brain: A Life in Neuroscience. Ecco Press.
Ghetti, S., & Bunge, S. A. (2012). Neural changes underlying the development of episodic memory during middle childhood. Developmental Cognitive Neuroscience, 2(4), 381-395.
Horowitz, L. M. (2013). Roger Sperry’s contributions to neuroscience. Journal of Neurophysiology, 109(4), 1003-1008.
Levy, J. (1985). Right brain, left brain: Fact and fiction. Psychology Today, 19(5), 38-44.
Nelson, K. (2007). Young Minds in Social Worlds: Experience, Meaning, and Memory. Harvard University Press.
Pinto, Y., Neville, D. A., Otten, M., Corballis, P. M., Lamme, V. A., de Haan, E. H., Foschi, N., & Fabri, M. (2017). Split brain: divided perception but undivided consciousness. Brain, 140(5), 1231-1237.
Springer, S. P., & Deutsch, G. (2021). Left Brain, Right Brain: Perspectives from Cognitive Neuroscience. W. H. Freeman.
Further Reading and Research
Recommended Articles
- Poldrack, R. A., & Farah, M. J. (2015). Progress and challenges in probing the human brain. Nature, 526(7573), 371-379.
- Bassett, D. S., & Sporns, O. (2017). Network neuroscience. Nature Neuroscience, 20(3), 353-364.
- Yarkoni, T., & Westfall, J. (2017). Choosing prediction over explanation in psychology: Lessons from machine learning. Perspectives on Psychological Science, 12(6), 1100-1122.
Suggested Books
- Gazzaniga, M. S., Ivry, R. B., & Mangun, G. R. (2019). Cognitive Neuroscience: The Biology of the Mind (5th ed.). W. W. Norton & Company.
- Comprehensive textbook covering all major neuroimaging techniques with detailed explanations of underlying neuroscience principles and research applications.
- Huettel, S. A., Song, A. W., & McCarthy, G. (2019). Functional Magnetic Resonance Imaging (3rd ed.). Sinauer Associates.
- Definitive technical guide to fMRI methodology, analysis techniques, and interpretation, suitable for researchers and advanced students.
- Luck, S. J. (2014). An Introduction to the Event-Related Potential Technique (2nd ed.). MIT Press.
- Essential resource for understanding ERP methodology and applications, with practical guidance for designing and analyzing EEG experiments.
Recommended Websites
- National Institute of Mental Health Neuroimaging
- Comprehensive information about current neuroimaging research, funding opportunities, and clinical applications from the leading mental health research institution.
- Society for Neuroscience Brain Facts (https://www.brainfacts.org/)
- Public education resource providing accessible explanations of neuroscience concepts, brain imaging techniques, and latest research findings.
- Organization for Human Brain Mapping (https://www.humanbrainmapping.org/)
- Professional organization offering educational resources, conference proceedings, and best practices for neuroimaging research methodology.
To cite this article please use:
Early Years TV Brain Scanning Techniques: Neuroimaging Windows into the Mind. Available at: https://www.earlyyears.tv/brain-scanning-techniques/ (Accessed: 28 October 2025).


